When a macaque has the choice between two lianasby Marie Delbasty and Julie Viana
Published by the May 10, 2021 on 8:00 AM
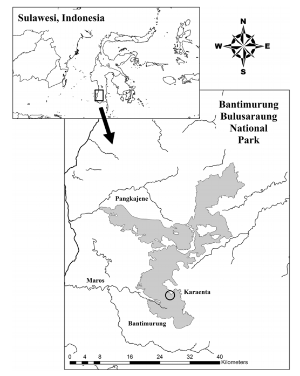
Two populations of moor macaques (Macaca maura) were studied in their natural environment in South Sulawesi, Indonesia in order to understand their use of two different habitats in karst forest.
Moor macaque is a species currently classified as "endangered" by the IUCN, mainly due to the disturbance and fragmentation of its habitat. That is why, in order to develop adequate conservation plans and management strategies, it is essential to study the patterns of habitat use in relation to the distribution of essential food resources.
Concerning the two types of habitat, they were characterized according to the vegetation present and its abundance as well as the topography and the presence of any trace of human activity.
The two habitats of this forest are in fact distinguished in two essential aspects:
The forest situated at the highest altitude with a steep slope, has few food resources but is not accessible to humans
vs.
The gently sloping forest, rich in resources but frequented by humans.
In order to carry out this study, each group of macaques was observed after having been accustomed to the presence of the scientists. The largest group consisted of about 30 individuals while the smallest group consisted of 18 macaques. The behaviour of the smallest group was studied from June to November 2016 while the largest group was studied from September 2014 to February 2015. Behavioural activities were defined as feeding, foraging, locomotion, social interactions and resting.
Although both habitats are used on a daily basis by each population, the analysis revealed that for both groups, the only behaviour that differed primarily between the two habitat types was time spent feeding. They spent more time feeding in the more food-rich forest habitat. The larger group spent more time overall in the food-rich forest while the smaller group spent more time in the food-poor forest.
The habitat with fewer resources is more of a refuge area for the macaques as they have no real predators and humans are the main threat. The larger group's use of a more productive but riskier habitat may be due to its history of provisioning, which may have allowed its individuals to have less fear of encountering humans. On the other hand, the individuals of the other group have never experienced provisioning. Another possible explanation is group size. Indeed, individuals in the larger group have less need to be vigilant because of their numbers, compared to the smaller group. In addition, the larger group might dominate the other group from a competitive point of view and thus be given priority for benefiting from resources.
In this context, macaques seem to be ecologically flexible, able to exploit the karst forest as a whole and to cope with human disturbance. It is important to protect the forest to allow the species to persist as habitat fragmentation threatens its survival. Thus, the management of this area would consist of balancing the needs of humans and macaques, and one of the solutions could be to educate local people on the protection of the species.
In a global context of loss of many species, the ideal would be to be able to leave in peace those for whom this is possible. Indeed, it seems preferable not to allow humans to access this area for the good of these macaques especially since this area is probably home to most of the remaining populations. Indeed, forest habitat with more food resources is a crucial part of the landscape for the survival of moor macaques in southern Sulawesi.
Thus, a question then arises:
would it be possible to let these macaques enjoy their habitats in peace while moving from one liana to another like Tarzan and Jane? To be continued…
Read the full study: Albani A., Cutini M., Germani L., Riley E. P., Oka Ngakan P. and Carosi M. (2020) Activity budget, home range, and habitat use of moor macaques (Macaca maura) in the karst forest of South Sulawesi, Indonesia. Primates 61, 673–684 (https://doi.org/10.1007/s10329-020-00811-8).

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.How to adapt your birdly behavior to the river flow?by Mireia Kohler Pacino and Oihana Olhasque
Published by the May 4, 2020 on 2:05 PM
The natural flow regime paradigm and the aim of study
In 1997, the natural flow regime paradigm has been established. This paradigm has become a real basement of management and basic biological study of running water ecosystems (Poff et al., 1997). This one establishes that the temporal variation in river flows requires the adaptation of structure and function of the aquatic ecosystems. To better understand this adaptation, many animals have been studied. In our case, the Cinclus Cinclus is chosen because of his large distribution in the world. We want to figure out if his behavior and energy use strategies are dictated by the natural river flow. We’ll use time-activity and time–energy budgets. In fact, it has proved to be a convenient approach to assess a bird's use of time and energy expenditure.
Using time-activity budget
Different behaviors of dippersTo answer to this question, the time-activity budget of the White-throated Dipper (Cinclus cinclus) was studied within a water basin in the Pyrenees, where natural flow regime is highly seasonal. To study the time activity budget, bird activities were categorized under four main headings: resting, foraging, diving and flying. In the study, between October 1998 and August 2001, birds activities were monitored each month using a portable tape recorder in combination with a telescope at a distance of 30–100 m. Overall, the analysis was made on 130 recordings: 62 males, 52 females, and 16 birds of unknown sex. As strategies could depend on external and river conditions, air temperature, water temperature and water column depth were measured on the behavioral surveys.
Parameters used in the study
Authors assumed that the Daily Energy Expenditure is calculated from an equation that includes time-energy budgets (obtained by incorporating time activity data), basal rate of metabolism, thermoregulation, locomotion, foraging, digestion, growth, reproduction, as well as all energy expenditures that eventually end up as heat production. The required foraging rate and the observed rate of energy gain were also calculated by dividing Daily Energy Expenditure with, respectively, the active day length for birds and the total time spent feeding by birds. Consequently, the ratio “Observed rate of energy gain” / “Required foraging rate” indicates how much faster observed feeding rates are in relation to minimum required feeding rates. For example, if birds gather food at a rate just enough to balance their energy budget then this ratio is equal to 1.
Parameters used in the DEE equationResults synthesis
The natural river flow is high during snowmelt (between April and June) and very low in summer. The behaviors are also chasing due to season: In winter our birds spend more time in foraging where food is rarely found and the water flow didn’t increase. In May, went the river flow increase, they have a rest for 70% of the day. Diving, flying and other activities showed no peculiar pattern, but there’s a relationship between water stage and time spent diving. Moreover, the ratios, observed rate of energy gain / required foraging rate indicated our birds could face high energy stress during winter but paradoxically none during high snowmelt spates when food is expected to be difficult to obtain. Unfortunately, the daily energy expenditure doesn’t seem to show any annual pattern. At this step of the study, they couldn’t find out whether Dippers use an energy strategy.
To go further....
With the actuals methods like calorimetry will be a complement to this study. To figure out, more information about dippers cycle life and potentials energy strategies. More generally, this study will serve the overwhelming challenge of maintaining native birds (especially those at risk) and more generally speaking biodiversity in human-altered rivers and streams.

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.Albatrosses: the laziest ocean birds?by Manon Yerle
Published by the July 8, 2019 on 9:51 AM
Movement ecology is a new scientific discipline that studies the movement paradigm in the animal kingdom. The fact is: How foragers choose their hunting technics? Foragers are animals that need searching in wild food resources. Since 1960s foraging theories are studied over the world. It’s known that free-handing animals must maximize their energy gain in order to spend as little as energy possible to find and catch their preys. To better understand how this works, some scientists conducted a study about the foraging strategy of the wandering albatross (Diomedea exulans). In this study they integrate instantaneous energy budgets within the movement ecology.

Wandering albatross (Diomedea exulans) in sit-and-wait (SAW) foraging strategy. Credit: John HarrisonMaybe, you have already heard “albatrosses are always sitting on water”, “albatrosses are lazy birds” …In fact when you see one, most of the time, it’s resting on surface of the water and does NOTHING! Why is it doing that whereas it could be attacked by sharks? Moreover, water is very cold! To answer at this primordial question, the foraging strategy of the wandering albatross was studied and well characterised in the Southern Indian Ocean. Four strategies are known: foraging-in-flight (FII), area-restricted-search (ARS), sit-and-wait (SAW) and resting (RES). Strategies depend on external conditions like weather features or wind, etc… In the study, between 2002 and 2005, during brooding periods, 45 birds were tracked but prey data capture were available only for 18 foraging trips. Over 18 birds, only 5 were studied because they were complete for all data.

Albatross in FII foraging strategy. Credit: John HarrisonAuthors assumed that net energy gain equal to energy gain minus energy expenditure. Energy gain is estimated by prey capture data (stomach temperature and digestion time) and conversion factors corresponding to the diet of the wandering albatross. Energy expenditure is estimated with continuous measures of heart rates values during trips. Finally, total trip net energy is estimated by cumulating instantaneous net energy gain along the trip. Assuming external factors (wind speed and angle between flight and wind) affects foraging, they implement the flying cost model to provide energy expenditure estimates.
The most used foraging strategy is sit-and-wait because this strategy is better for the brooding period; they obtained higher net energetic gain when foraging trips are short. So, albatrosses aren’t lazy, but they are searching for food. Are their results available regarding fewer numbers of individuals? In statistical analyses it is assumed that results are available if the number of individuals is higher than 30, which is not the case here. Moreover, optimal models don’t work in wild life because there is always external factors that prevent it.
Whereas previous studies (Weimerskirch et al. 2005) identified FII strategy as the most optimal for long trips, our study identified another strategy for shorts trips, SAW. In fact, birds need to provision chicks frequently and that requires more energy than during incubation. Breeding stage defines the foraging strategy used. Another study should be managed during the incubation period implementing an instantaneous energy-budget model. Moreover, they should implement internal factors as thermoregulation that is more important in SAW for example. Now, we know how wandering albatrosses choose their foraging strategies. We can ask if SAW really is a good foraging strategy because albatrosses are more vulnerable against predators like tiger sharks.


This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.Effects of an exotic prey species on a native specialist: Example of the snail kiteby Mathieu Finkler and Hyppolyte Terrones
Published by the September 11, 2018 on 12:34 PM
Exotic species have largely been studied over the years, their effects on native populations, their consequences... Most of the studies aim to see the competition between a native species and an exotic one. Here the study focus on the effect of an exotic species on a native predator.
Florida snail kite (Rostrhamus sociabilis plumbeus) are endangered, their populations are drastically declining in recent years. It is important to study them and to determine why their numbers are falling to implement an adapted conservation strategy.
The purpose of the study is to assess the effects of the recently introduced island apple snail (Pomacea insularum) on snail kite behavior and energetics comparing with the native prey (Pomacea paludosa).

Juvenile snail kite - Cláudio Dias Timm - CC BY-NC-SA 2.0The authors determined different parameters such as the proportion of snail dropped, the searching and handling time, the consumption rate and proportion of time in flight. Caloric intake of both species has been determined by a model (Sykes 1987) and so is the daily energetic expenditure. Caloric balance seems to be perfectly suitable in this case because the difference in intake calories could affect all the life history traits and be the cause of the fast decline of the kites.
Foraging on exotic snails led to a greater proportion of snails dropped, a lower consumption rate, a longer handling time and a lower energy balance (figure below). These conclusions are particularly true and worrying for juveniles. This results indicates that feeding on exotic snails will decrease their energy and so less energy will be available for others activities (like reproduction, growth, defence against predators...). Finally, lakes where only exotic species are present (Tohopekaliga) could form an ecological trap.
From Cattau et al. 2010Even after this study, it will be hard to conclude on an optimal foraging theory because both snail species were never found together in a lake. Therefore it could be interesting to make the same study in a lake were both species are present. Furthermore, this study has been conducted during the breeding period. During breeding period, species will need more energy to feed their offspring, to protect them, potentially leading to a greater difference in energetic balance.
Further studies may focus on the fact that kites feed on larger exotic preys (compared to native preys). Are the smallest individuals not available for kites or do kites choose to feed on larger exotic preys to compensate for their lower energetic content ?
This method could be used in others studies of trophic relationships and not only on native-exotic conflict. For example if human overfish a species, the predator of this species will have to change preys. So it will be important to calculate the energetic balance with the new prey.

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.Are vulture restaurants needed to sustain the densest breeding population of the African white-backed vulture?by Mikel Cherbero and Tom Laffleur
Published by the August 3, 2018 on 10:00 AM
As obligate scavengers, vultures are entirely dependent on carrion. These last decades, carrion abundance has decreased in many areas. The two main causes of this trend are clearly identified. Natural habitat destruction reduces wild animal carrion abundance, which is the natural resource of scavengers. At the same time, the modification of agricultural practices, essentially the generalization of carcass rendering, has reduced the availability of cattle carrion. These factors have led to a negative trend on scavenger populations. This is especially the case in Africa, where most of avian scavenger species are now endangered. African savanna ecosystems were originally rich in avian scavengers, but most of the species are actually endangered.
_on_zebra_carcass_..._(33282103735)_201x301.jpg)
White-backed vultures feeding on zebra carrion - Bernard Dupont - CC BY-SA 2.0In this study, authors model the carrion ecology of an ecosystem in Swaziland which is home to the densest breeding population of the African white-backed vulture (Gyps africanus), a critically endangered species. They also study other threatened scavenger species of Swaziland: white-headed vulture (Trigonoceps occipitalis), Nubian vulture (Torgos tracheliotos), marabou stork (Leptoptilos crumenifer), tawny eagle (Aquila rapax) and bateleur (Terathopius ecaudatus). The purpose of this work is to better understand the feeding activity of the white backed vulture and to modelize population trends for these six species (using life-history traits and modelization of carrion availability), and based on these results authors discuss if the establishment of vulture restaurants would be beneficious.
They first calculated the foraging radius (r) of the white-backed vulture, based on the Foraging radius concept theory. The foraging radius represents the radial distance from the nest in which the energy inputs are greater than the costs of feeding and needs of the vulture and its litter. This theory is adapted to this species, because vulture always comes back to the nest after feeding. They compiled available bibliography and collected data on metabolism and life-history parameters of the species. Using this data, they applied a model created with the same purpose by Ruxton & Houston in 2002 for the Ruppell’s vulture (Gyps rueppellii), which is phylogenetically and ecologically close to the white backed vulture.
The results shows that the foraging radius is 260 km in the main part of the year. This radius is large, vultures can feed in neighboring countries (South Africa, Mozambique), it implies an international cooperation in the management of these endangered populations. A positive aspect is that individuals can spread over large area, so the studied population can form or sustain other populations. On the other hand this radius is much greater than the natural reserve surface, thus vultures can be exposed to several risks, like poisoning, when they are feeding. When vulture have to feed a chick, energy needs are logically greater so the foraging radius is reduced to 40 km. Carrion availability is more problematic during this period, which should therefore be targeted if vulture restaurants are setted up.
Using novel Population Dynamics P-Systems, they show that carrion provided by wild ungulates biomass is currently enough to sustain this vulture species. According to the model, white-backed vulture population will continue increasing in Swaziland, and will pass from approximately 300 pairs to more than 500 in twenty years. The other studied avian scavenger populations will follow the same trend, but are far less abundant than white-backed vulture. The model shows also that three main species are composing vultures’ food: the Impala (Aepyceros melampus), the blue wildebeest (Connochaetes taurinus) and the plains zebra (Equus burchelli) represent 55 % of total carrion.
However, in light of the forecasted population increases, food will become a limiting factor. This is particularly true for the period from November to April, for which the model show a carrion deficit. During this period African vultures are not breeding so they can go far away to feed themselves. But the model also shows a carrion deficit during the breeding season after five to thirteen years of simulation. This lack of food resources can be considered as a natural limiting factor. According to the model, the area has probably reach its maximum carrying capacity after twenty years.
To conclude, authors suggest that setting up supplementary feeding stations in Swaziland should be seriously considered, especially during the breeding season. Good managed restaurants would have several advantages : secure the viability of the population and thus increase its capacity to act as a source population, avoid poisoning risks and create the opportunity to capture and tag vultures. This last point would allow to improve knowledge about the avian scavenger species, necessary for a more effective conservatory management.

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Can bioenergetic models help the re-introduction of the native Rio Grande cutthroat trout in a Southwestern headwater stream?by Emmanuel Bourgoin and Aurélien Callens.
Published by the May 16, 2018 on 1:47 PM
Re-introduction of native species is far from being simple: many parameters must be accounted for! To illustrate that, we are going to take a closer look at a study made by Kalb and Huntsman (2017) on a stream in southcentral New Mexico which was deemed suitable for re-introduction of the native Rio Grande cutthroat trout (Oncorhynchus clarkii virginalis). Before re-introducing this species, researchers wanted to know if the habitat was able to sustain it. Thus, they evaluated habitat using resource selection functions with a mechanistic drift-foraging model to explain rainbow trout distributions. They studied rainbow trouts because they are present on the stream and are close relative to the Rio grande cutthroat trout, consequently all the results of this study can be extended to this native species.

Rainbow trout - Timothy Knepp/U.S. Fish and Wildlife Service - Public domainEach month, the available habitat and foraging locations were evaluated along the stream. Foraging locations were defined as the location where they could observe a foraging fish. For each foraging site, the length of the fish was estimated and physical characteristics such as discharge, focal velocity (current velocity at the head of the fish), depth, cover distance and temperature were measured on the exact fish location. These parameters were also measured on the available sites. Macroinvertebrate drift was estimated on all the locations (available and foraging). All these parameters were used in bioenergetic models which allow the researchers to estimate all the intakes of the fish (net energy intake, energy assimilated…) and all the costs associated with foraging (capturing a prey, swimming…).
First, they observed that macroinvertebrate drift was strongly season- and temperature-dependant with high values in summer and fall and low values in winter and spring. Moreover, as we must expect it, water temperature, depth and discharge were found to be seasonal parameters too. Secondly, models identified the depth as the most limiting factor for habitat selection: trout of all ages preferred habitat location with a greater depth. The most interesting thing about the models is that they can show the characteristics of the chosen habitat according to the age of the trout and the season. In fact, they showed that during the winter the smaller size-classes were more likely to choose a position closer to cover. Additionally, they highlighted that spring was the season with the greater energy intake for all the size-classes expect the 4+. Finally, drift-foraging models identified that 81% of observed trout selected positions could meet maintenance levels throughout the year and 40% of selected habitats could sustain maximum growth. Despite these last observations, the larger size-classes were energetically more limited throughout the year.
This study showed that trout population prefers deep pool habitats with slow moving water and that this stream was able to sustain a great population of rainbow trout and could consequently sustain a great native population of Rio grande cutthroat trout. However, authors warn us about the risk of hybridization and interspecific competition and suggest removing the non-native fishes first.
To answer the question in the title: yes, bioenergetic models can help to re-introduce a native species in a given environment. Nonetheless, this example is really specific: author had the chance to find and study a close relative to the native trout in the stream! The main thing to remember is that bioenergetic models give a lot of useful information on how a species uses an habitat and must be taken into account (if applicable) in the management of species.

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.