Corals and algae, a relationship in danger: a model to predict their future! by Clara Dignan, Anna Gago and Anabelle Leblond
Published by the March 2, 2020 on 2:14 PM

Bleached branching coral (foreground) and normal branching coral (background). Keppel Islands, Great Barrier ReefCorals that come together to form coral reefs are shelter to 25% of our planet's marine life according to the WWF. This biodiversity is fundamental. It’s both a source of income and food, and it provides irreplaceable services to humanity. But today coral reefs are in danger. They are directly threatened by global warming. In forty years, 40% of the reefs have already disappeared and scientists agree that if nothing is done by 2050, all of them will be gone (Coral guardian).
Coral polyps and algae, an endosymbiotic relationship
Coral bleaching has now become a major global concern for the future of coral reefs. Temperature rise appears to be one of the main causes of bleaching, affecting growth, feeding and other ecological processes on reefs. This bleaching phenomenon is due to the expulsion of zooxanthellae, the symbiotic microalgae living in the tissues of the polyps (the coral is made up of a colony of polyps that participate in the making of its skeleton). These unicellular algae carry out photosynthesis and provide, for the most part, the energy that corals need to develop and grow. Exchanges between the polyp and the zooxanthellae mainly concern nitrogen, phosphorus, carbon and biosynthetic intermediates. The presence of zooxanthellae being responsible for the color of the colonies, bleaching is therefore the symptom of a coral which is no longer in symbiosis, which generally results in the death of the coral.
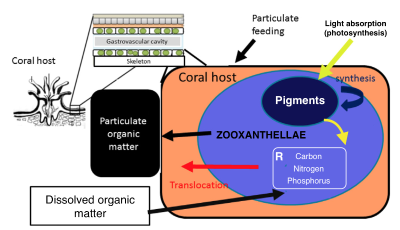
The coral-symbiont relationship and its interaction with the overlying water column.Prediction models
Since few year, scientists analyze corals and try to predict their bleaching evolution. In this aim, a collaboration between several organizations such as CSIRO have set up a first hydrodynamic, sedimentary and biogeochemical model called: « eReef ». This model simulates the environmental conditions as the temperature, the background light and the organic nutrient concentration of the Great Barrier Reef at several scales. It allows accurate prediction of factors influencing coral processes from satellite remote sensing images.
However, for more representative modelling, it is necessary to apply models that take into account the coral-symbiont relationship and the stress related to environmental variations. In this framework, Baird et al. have developed a model which, in parallel to the environmental conditions obtained from the « eReef » model, also takes into account essential parameters in the symbiotic process such as biomass and growth rate of zooxanthellae, pigment concentration, nutritional status as well as tolerance characteristics such as sensitivity to reactive oxygen concentration (oxidative stress).
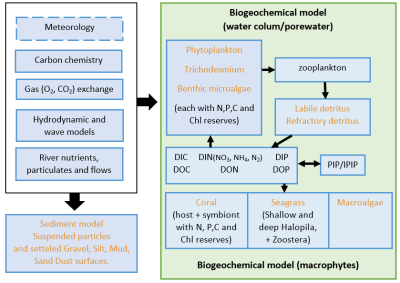
The eReefs coupled hydrodynamic, sediment, optical, biogeochemical model. Orange labels represent components that either scatter or absorb light levels. (For a better understanding of the colour used and the abbreviations, the reader is referred to the web version of the article)Take home message
This coral bleaching model applied under realistic environmental conditions has the potential to generate more detailed predictions than satellite coral bleaching measurements. In addition to predicting coral bleaching, this model will now make it possible to evaluate management strategies, such as the introduction of temperature-tolerant individuals or species or localized shading.
Nevertheless, this model is still too simplistic to make real predictions. It is based only on the process of a single type of coral and macro-algae and does not take into account all phenomena related to bleaching. It is therefore seen as a step forward for science that could allow for future reevaluations of the effects of bleaching.
Bibliography
A new approach of modeling dissolved organic matter release by phytoplankton. Is it an improvement?by Bastien Mourguiart and Thomas Panarotto
Published by the April 12, 2019 on 6:53 PM
Phytoplankton is playing key roles in marine ecosystems. These microscopic plants are known, in particular, to be a part of the “Biological Pump”. Using photosynthesis as metabolism, it fixes carbon of the atmosphere to produce energy. This process reduces atmospheric concentration of CO2 and limits the greenhouse effect. It also produces oxygen indispensable to the life of many organisms.
Phytoplankton forms the basis of the marine food chain. Autotrophic organisms, they convert sunlight energy into chemical energy (food). This food constituted by molecules with carbon (organic matter) can pass directly along the food chain when zooplankton eats the phytoplankton and in turn are consumed by larger animals such as fishes, whales, squids, shellfishes and birds. Organic matter (OM) can also be released by phytoplankton in a dissolved form named dissolved organic matter (DOM). Organic matter can then be absorbed by bacteria and enter the main food chain when bacteria are eaten by zooplankton.
Heterotrophic prokaryotes (all animals) use carbon contained in DOM as a major source of energy. So, products excreted by phytoplankton are really important in the functioning of marine ecosystems and understand how DOM is released in the environment is essential.
Livanou et al. present in their article “A DEB-based approach of modeling dissolved organic matter release by phytoplankton” a new model to calculate DOM release by phytoplankton. They apply Dynamic Energy Budget (DEB) theory on phytoplankton cells for that. In this study, the metabolism theory leads to describe DOM fluxes, based on assumptions about energy uptake, storage, and utilization of N and C. The authors are mainly interested in how DOM is excreted by phytoplankton under different nitrate concentrations.
They calibrate and test the goodness of fit of the model using past laboratory data. In this previous experiment, others scientists (Flynn et al. 2008), measured DOM released by one species of phytoplankton with two phase of nutrient concentration: one with enough nitrate for all the individuals and one with nitrate in limitation. The results of DEB-Model fit well to experimental data according to Livanou et al. even it does not explain all the information: in the figure below, lines (the model) do not fit exactly the points (experimental data).
Figure 2 in Livanou et al.To conclude, they explain quickly that their model permit to describe how DOM is released. In no N-limitation condition, passive mode is used and DOM excreted is more accessible for bacteria. For N-limitation condition, DOM released cannot be used by bacteria and it tends to accumulate.
This study is maybe a step forward in comprehension of phytoplankton physiological mechanisms. However, in our opinion, it is not really useful to improve our understanding of energetic flows in the oceans. Moreover, the model was calibrated for only one of the thousand species of phytoplankton existing in nature. It should be calibrated for others species to catch up more processes which can change between species. The model can be more accurate catching up all the processes in this particular species too: the fitting test shows some differences from the experimental data (Figure 2). There is also limiting by the fact that only one nutrient is used as limiting nutrient: in reality, there can be more (Moore et al. 2013). To summarize, it needs very lot of work on this model to employ it in real ecosystems and be an improvement.
Other references:

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.High throughput screening methods to assess pollutants effects: A relevant technique?by Alexandre Bijaye and Melody Fernandez
Published by the March 12, 2019 on 6:43 PM
The purpose of the paper written by Miller et al. in 2016 is to assess the effectiveness of HTS (High Throughput Screening) techniques to predict the effects of metal nanoparticles on a population of Isochrysis Galbana, a common species of phytoplankton.
High Throughout Screening is one of the newest techniques used in toxicology and which is planned to be implemented in biological and chemical sciences in the near future. Their technology is based on the analyzes of chemical compounds to be conducted in a short time. The affinity of biological structures which is related to the toxicity to be defined.
In this article, we’ll focus on nanoparticles: Engineered nanoparticles (ENPs) are actually an emerging form of metal contamination. These particles are widely used in biochemistry, engineering,… Four elements are studied in this paper: Ag, ZnO, CeO2 and CuO. The studied concentrations of each compound chosen were known to affect phytoplankton populations.

Silver nanoparticules (T. Theivasanthi/Wikimedia)The results showing a decline of the photosynthetic activity (PSII) are compared to the HTS tests results. DEBtox models are energycally and toxycodynamically balanced modelling techniques (DEBtox and TD) and are here used to evaluate the impact of ENPs.
First, the decline in photosynthetic activity is a good predictor. Phytoplankton are vulnerable to pollution, particularly because of their aptitude to accumulate contaminants. As a result, such a bioaccumulation can impact food webs integrity.
Isochrysis Galbana populations were cultured at 20°C in sterile seawater (at 34 per thousand of salinity). In these conditions, HTS techniques measured cellular lesions responding to a toxic agent, permitting here to measure the potential impacts of ENPs on organisms.
Four HTS tests were undertaken (based on the mitochondrial membrane potential, ROS occurence, (3) cellular efflux pump action and cell membranes permeability). These tests are based on fluorescence and cell health. The impact of metallic nanoparticles was also measured. However, the results obtained were not consistent enough to highlight responses to ENP exposure:
Non-HTS tests were performed by the measurement of the impact on photosynthetic efficiency. The metal concentrations were measured using the graphite furnace atomic absorption.
Fluorescence kinetics of chlorophyll was also measured with an amplitude modulated pulsed fluorometer. Then the maximum fluorescence yield was computed using WinControl Software. This the maximum fluorescence variation is assumed to be a measurement of the potential quantum yield of the PSII.
A clear decrease in the performance of the PSII was observed for all the ENP’s:
- A greater decrease was observed for ZnO
- Also, a notable decrease was measured for CuO, at low concentrations
- Finally, the presence CeO and Ag decreased the performances as well even though the decrease was the smoothest.
According to the authors, these results linked with the different dissolution rates that are specific to each compound. In this direction, Zn has a high dissolution rate, CuO and Ag dissolve very slowly and CeO has an undetectable dissolution rate.
As a conclusion by the authors, HTS methods can not be used to measure the impacts of metal particles because of their poor predictive power. So far, traditional ecotoxicological methods must be used.
With such results in mind, we also agree on this statement: the four tests led by the authors all brought different conclusions. As HTS methods rely on the affinity of biological structures which is often related to toxicity to be defined, the presence of cellular compensatory mechanisms could have masked cellular responses to ENPs expositions.
In conclusion, although HTS methods offer promising opportunities in the biological sciences (such as ecotoxicology), there are need for further investigation in this area before these techniques can be commonly adopted and used.
Cited study: Miller, Robert J., et al. (2016) Photosynthetic efficiency predicts toxic effects of metal nanomaterials in phytoplankton, Aquatic toxicology 183, 85-93.

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.Time and concentration dependency in the potentially affected fraction of species: The case of hydrogen peroxide treatment of ballast waterby Marie-Adèle Dutertre and Maud Hautier
Published by the December 10, 2018 on 4:32 PM
Globalization and international trade made natural gates easier to cross for species. As a consequence, few species were able to travel long distance and settled in new habitats where they become invasive species.
More than 80% of industrialized goods in the world are transported by the oceans in container ships. In many cases, container ships is discharged in the destination port and go back empty. Whereas, the structure of this kind of ship does not allow them to travel empty and with stability. This is for a problem of stability that ballast exists. Since the 19th century, ballast with rocks was substitute with water. Before ships leave the port , water is loading in tank and at the destination port tanks are discharged.
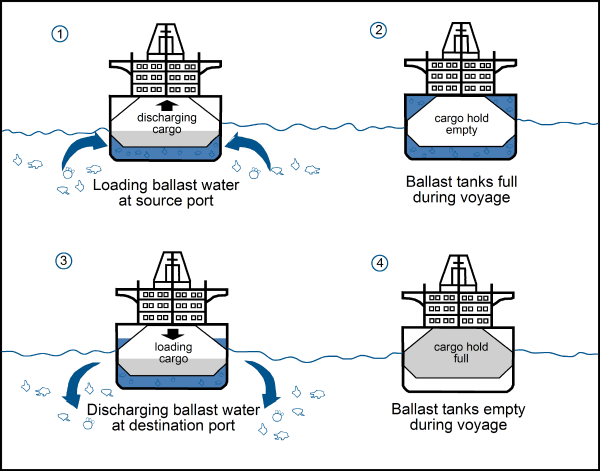
http://www.seos-project.eu/modules/marinepollution/marinepollution-c04-p05.fr.htmlBallast water transport contribute to invasive species spreading. In order to fight against exotics species, waters ballast are treated with Hydrogen Peroxide (H2O2). But there is a question : how to be sure that ballast water is effective and is not toxic for the marine environment ? In order to evaluate the environmental impact of the treatment, a study has been conducted. Three taxa has been chosen, among them, two crustacean, two algae and one rotifera : C. volutator, A. salina, E. costatum, D. teriolecta, B. plicatilis. The authors of the study consider three dimensions : Hydrogen Peroxyde concentration, the effect of the Peroxide Hydrogen on organism and Hydrogen Peroxide exposure time. In the experiment, they made the tree dimensions varied and they considered as the final aim, the mortality, the immobility and the inactivation of the organism. The results are used in a mechanistic model which is based on the description of Dynamic Energy Budget theory. The DEB theory consists of a simple set of rules that specifies how organisms acquire energy and building blocks from their environment to fuel their life cycle. It is used to rely the observed effects and the hydrogen peroxide concentration in the experiments. The DEB-tox model allows to determine ECx — Effect Concentration — : the concentration which induces a response of x% between the baseline and maximum after a specified exposure time ; and the HCx — Hazardous Concentration — : the concentration which is dangerous for x% of the population. Thanks to this values, it is possible to determine the PAF — Potentially Affected Population— with means the part of an ecosystem potentially affected by a drug concentration. The results show an interspecific response variability with means different interspecific H2O2 sensibility. Sensibility is a combination between time exposure and the concentration. The conclusion of the study is that the hydrogen peroxide is effective for treating ballast water.
Concentrations, effects and time exposure were studied there. The choice of the five species is a wise choice as a result of the representativeness of a wide selection of sensibility which allows to extrapolate this results to other species and then estimate the effect of hydrogen peroxide treatment on other species present in water ballast. Whereas the aim of the study was to assess environmental risks of hydrogen peroxide treatment, and the obtained results here cannot be used to conclude regarding as the environmental risks.
To assess more precisely the risk, it is important to consider the hydrogen peroxide degradation and its potential impact on marine ecosystem. The H2O2 is oxygenated water which would rapidly be decomposed : 2H2O2 => 2H2O + O2. In this case, the hydrogen peroxide would not impact the environment.
Furthermore, sub-lethal effects are sufficient to reduce the viability of the organisms and for that, lower concentration of H2O2 and lower time exposure are sufficient. The purpose is to neutralize exotic species with lower environmental and economic costs. Moreover, in order to reduce again the hydrogen peroxide used quantity, other studies show the efficiency of using UV, Ozone, and ultrasound for neutralizing species. The hydrogen peroxide treatment can also be used with alkaline water which allows to obtain the same result with lower concentration and time exposure.
An other option is to establish regulated areas for discharging and to filter and to purify ballast water before discharging in the environment.

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.





