Pyramids, built by the Egyptians and reversed by sharksby Pierre Labourgade, Valentin Santanbien and Morgan Schler
Published by the October 5, 2020 on 8:28 AM
The case of a extreme inverted trophic pyramid of reef sharks supported by spawning groupers in Fakarava, French Polynesia
Predators play a key role in the structure and functioning of ecosystems (Paine 1966; Begon et al. 2006). Through food webs, the relationship between preys and predators is crucial in order to maintain a balance, including in marine ecosystems (Woodson et al. 2018). A trophic pyramid is a graphic representation designed to show the biomass at each level of the food chain. The lowest level starts with decomposers and the pyramid ends with top predators. This is called a pyramid because generally, the biomass in the lower levels turns out to be much higher than in the upper levels (Figure 1 A). However, in the marine environment, and in some remote and almost unoccupied areas, predators may dominate in terms of biomass, generating an inverted pyramid (Figure 1 B).
Figure 1 Diagram of a normal (A) and inverted (B) trophic pyramidAggregations of grey reef sharks, Carcharhinus amblyrhynchos are observed on some reefs in the Indo-Pacific (Robbins 2006) (Figure 2). The southern pass of Fakarava atoll in French Polynesia has a population of around 600 individuals of this species (Mourier et al. 2016) (Figure 3). This makes it one of the few places to present such a large grouping. With such a large population on a reef channel of just over 1 kilometer, the area has up to three times the biomass per hectare documented for any other reef shark aggregation (Nadon et al. 2012). The biomass of predators is then much greater than preys, thus generating an inverted trophic pyramid. During this study, scientists tried to understand how those large group of sharks can survive when prey biomass is insufficient.

Figure 2. Aggregation of grey reef sharks
Figure 3. Panoramic view of Fakarava atollDuring the study period, video-assisted underwater visual surveys conducted across the pass allow the researchers to find that sharks population can represent up to 700 individuals. Then, scientists use bioenergetic models based on known value of parameters that influence energetics needs of shark-like “asymptotic length”, “growth rate” or “proportion of fish in the diet” to determine prey biomass needed for all the individuals. According to bioenergetic models, the food requirements to maintain that large population is approximately 90 tons of fish per year, which is not provided by the environment as it is. However, the pass is used as a breeding ground for many fish species, thereby reducing the prey-shark ratio. This means that the prey biomass will be much higher than that of sharks during these reproduction periods (Mourier et al. 2016), leading to frenetic predation behavior in the shark that will allow it to meet its energy needs (Robbins and Renaud 2016; Weideli, Mourier, and Planes 2015). Furthermore, the continuous presence of prey aggregation is ensured by the successive migration of different species to this site, in order to meet the metabolic demands of the shark population present (Craig 1998). With simulation-based on researcher bioenergetic model, sharks would not have enough energetic income after 75 days if other prey species didn’t migrate to the pass. There is, therefore, an idea of metapopulation where the exchange of individuals between populations in normal and inverted trophic pyramids ensures that the energy needs of each individual are met (Figure 4). This exchange of individuals between populations will allow the long-term maintenance of the species and, in the case presented here, of the shark.
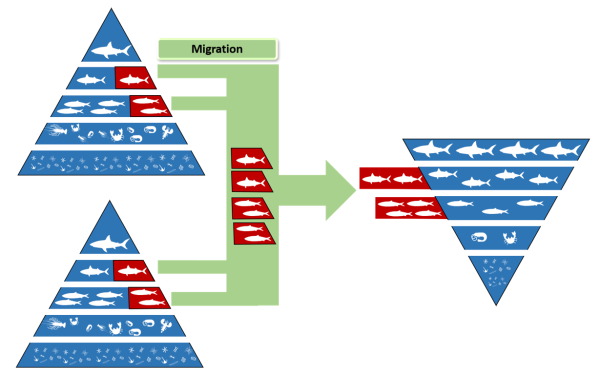
Figure 3. Diagram of the transfer of potential prey for the shark between two normal pyramids and one inverted trophic pyramid via migratory flowsThe temporal aspect in the movement of individuals between populations is therefore important to be considered during the development of management and conservation measures. Indeed, if we want to ensure the sustainability of the grey reef shark in this pass, we must not only protect the habitat on-site, but also the original habitat of different species that come to reproduce in the pass. These species are indeed essential for the survival of sharks since they represent the only source of energy available during certain periods of the year.
Other cited articles:

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.Spatial response of plaice and sole to climate changeby Arnaud Dupond and Alix Pascal
Published by the August 3, 2020 on 7:54 AM
Scientists admit that climate change is one of the main driving forces which change species distribution and abundance in many ecosystems.
In this case, a modification of abiotic variables can affect the “niche concept” and can also change species geographical distributions. For the marine environment, this notion of geographical dependence is important. Indeed, marine organisms show several distinct stages during their life and each of these stages evolve in a specific habitat.
The objective of this study was to use different models based on physiological aspects and environmental variables in order to estimate habitat occupation by plaice and sole under different climate change scenarios in the North Sea.
How to reach this objective?
To predict new habitats, researchers considered environmental variables and determined their effect on the food web, but also the effect of the food web on the water chemistry. To do that, the ecosystem model used functional groups of taxa. Their taxa are phytoplanktonic, planktonic and macrobenthic organisms. Some of them have a direct effect on the water chemistry and are regulated by other taxa. The food web is also used to quantify the availability of the habitat’s resources. Thanks, of this two types of model results fit more precisely reality of the distribution.
The sample strategy is built like this, data are collected daily on surface of 10x10km sin the North Sea.

Figure 1: Schematic simplification of the models used in the studiesResults of the study
The model of environmental variables shows the predictions for temperature and food conditions between 1989 and 2002. This data showed benthic production is concentrated along the southern coast during the year 1989, whereas in 2002 is concentrated in the Southern bight (figure 2).
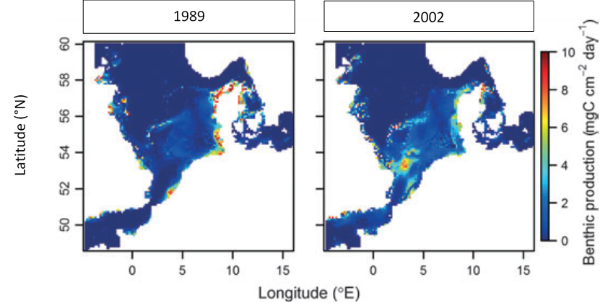
Figure 2: Comparison of the benthic production between 1989 and 2002 in the North SeaAn important fact is that the temperature rate inside of which the growth is positive will change with the size of the fish and according to abundance of food (the more food is abundant the higher rate of temperature is). Indeed, bigger fish need higher temperature to grow optimally. Figure 3 defines the areas of maximum potential daily growth of each class size of plaices in 1989 at the left, and in 2002 at the right.
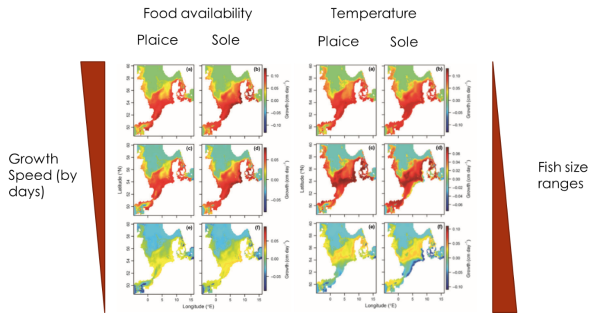
Figure 3: Comparison between the three size ranges of plaices and soles, of the speed growth in regard of two environmental parameters, the food availability and the temperatureThe results for maximum potential growth per day seem to give the same result as the estimate of average abundance. (Figure 4).

Figure 4: Comparison of the plaice and sole abundance distribution in the North SeaConclusion
For the plaice, migrations during different stages of life maximize their physiological performance during the summer season, in the winter, the adult’s distribution is determined by the best spawning habitat and shows maximisation of their fitness. Sole differs in their physiological traits and have a higher optimal growth temperature which explains the difference in life habitat. As for plaice, the area indicated high quality habitat for the different size class.
This study can predict the evolution of species distribution with a model of environmental changes and one of physiological changes but in our case, we can just explain data collected not the prediction made with the model.
Read the full study: Teal, L.R., van Hal, R., van Kooten, T., Ruardij, P. and Rijnsdorp, A.D. (2012), Bio‐energetics underpins the spatial response of North Sea plaice (Pleuronectes platessa L.) and sole (Solea solea L.) to climate change. Glob Change Biol, 18, 3291-3305. https://doi.org/10.1111/j.1365-2486.2012.02795.x

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.A little bit of salt and heat... a good recipe for goby metabolism?by Maxime Deau, Quentin Garreau and Dorian Raoux
Published by the June 1, 2020 on 2:18 PM
As the literature shows, a variety of factors influence the well-being of fish populations. For example, we know that some fish may or may not be very sensitive to changes in the conditions of their living environment (water temperature or salinity). These changes can affect their metabolism (reduced fertility, growth, etc.) or even, in the worst case, lead to the death of individuals. The goby (Pomatoschistusmicrops) (Figure 1), a relatively tolerant species and an essential central link in the food web is one of the species studied in the observation of the impact of these changes on the fish fauna in the Minho estuary in Portugal (Figure 2).
In this study, the researchers were able to model the evolutionary dynamics of p.microps populations based on models that take into account different parameters of goby's life cycle like fertility, mortality, migration rate and the effect of environmental parameters such as salinity and temperature. The aim of these models is to describe the evolution of the different life stages of this fish by establishing the possible impacts of climate change on their metabolism. In this framework, they studied both the impacts of temperature and salinity and combined the impact of both.
It has been noted that salinity directly influences the metabolism of individuals. Indeed, it plays a particular role in the survival of many aquatic organisms but also on their growth (strong allocation of energy to osmoregulation; Rigal, F. and al.,2008). Therefore, it plays a role in the growth of the goby as well as indirectly on its prey. The latter will be less available, which implies a higher energy expenditure for predation. However, this species resists large variations in salinity (0 to 51 psu). For temperatures, the impact is more diverse. Since the goby does not thermoregulate, its metabolism is directly influenced by the temperature of the environment. In addition to its significant effect on pregnancy, it also has an impact on migration, reproduction, recruitment and mortality. (Sogard, 1997; Hurst et al., 2000; Hales and Able,2001; Hurst, 2007; Jones and Miller, 1966; Claridge et al.,1985; Wiederholm, 1987).
Regarding the goby’s responses to these parameters, the research team has implemented them in the model, running different scenarios (salinity and temperature variations). Temperature and salinity variations studied separately led to population crash, except for a salinity lower than the current state ( -5psu). However, the combination of the two variables gave scenarios showing an increase in the population when the salinity was -5 psu, with temperatures ranging from +1 to +3°C, with an optimum at +2°C (see figure 5).
For example, in extreme temperatures, the fish activity will be greatly reduced, which will imply a decrease in the search for preys or sexual partners, causing feeding and mating problems (predation of eggs by males (Magnhagen, 1992). However, a slight increase in temperature could cause a longer reproduction period, allowing for a greater number of offspring to be generated. It has also been noted that with an increase in temperature, there is a delay in the breeding period, leading to the appearance of offspring in a period that may be less favorable for their proper development (early winter/lower metabolism).In conclusion, climate change, through its effects on water temperature and salinity, will have a significant impact on common goby populations. Indeed, these parameters have a great influence on the metabolism of these fish (whatever their stage of development).In many scenarios, increases in temperature and salinity can cause crash populations. But beware, in some cases (increase in temperature and decrease in salinity) the population of Pomatoschistus microps would tend to increase. Even if this scenario seems favorable for this species, some others will suffer. in fact, a study conducted on Arctic fish species has confirmed these trends
It is therefore clear that climate change affects population dynamics by changing fish environment and impacting their metabolism. It’s therefore important to continue this kind of study to have a better idea of these repercussion on a global scale. We are largely responsible for climate change, so it is up to us to make sure that we limit our impacts. Here is a link that will teach you how to reduce your carbon footprint through 20 examples of simple everyday actions: http://www.globalstewards.org/reduce-carbon-footprint.htm
Other cited studies:
Claridge, P.N., Hardisty,M.W., Potter, I.C., Williams, C.V., 1985. Abundance, life history and ligulosis in the Gobies (Teleostei) of the inner Severn Estuary. J.Mar. Biol. Assoc. U. K. 65, 951–968.
Hales, L.S., Able, K.W., 2001.Winter mortality, growth, and behavior of young-of-the-year of four coastal fishes in New Jersey (USA) waters. Mar. Biol. 139, 45–54.
Hurst, T., 2007. Causes and consequences of winter mortality in fishes. J. Fish Biol. 71, 315–345.
Hurst, T.P., Schultz, E.T., Conover, D.O., 2000. Seasonal energy dynamics of young of the year Hudson River striped bass. Trans. Am. Fish. Soc. Taylor & Francis 129, 145–157.
Jones, D., Miller, P.J., 1966. Seasonal migrations of the common Goby, Pomatoschistus microps (Kroyer), in Morecambe Bay and elsewhere. Hydrobiologia 27, 515–528.
Magnhagen, C., 1992. Alternative reproductive behaviour in the common goby, Pomatoschistus microps: an ontogenetic gradient? Anim. Behav. 44, 182–184.
Rigal, F., Chevalier, T., Lorin-Nebel, C., Charmantier, G., Tomasini, J.-A., Aujoulat, F., Berrebi, P., 2008. Osmoregulation as a potential factor for the differential distribution of two cryptic gobiid species, Pomatoschistus microps and P. marmoratus in French Mediterranean lagoons. Sci. Mar. 72, 469–476.
Sogard, S.M., 1997. Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull. Mar. Sci. 60, 1129–1157.
Wiederholm, A.-M., 1987. Distribution of Pomatoschistus minutus and P. microps (Gobiidae, Pisces) in the Bothnian Sea: importance of salinity and temperature.Memoranda Societatis pro fauna et flora Fennica 63, 56–62.
This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.Shad, those endangered travelersby Alicia Dragotta and Claire Valleteau
Published by the April 6, 2020 on 1:52 PM

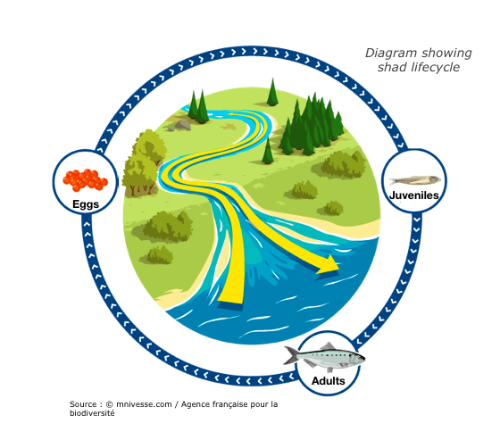
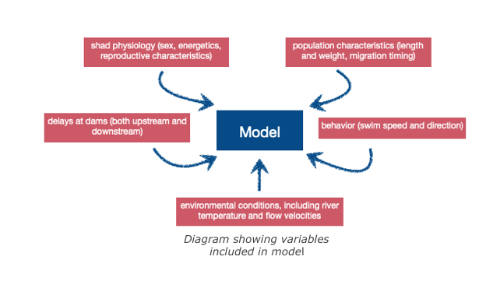
Photograph by MRM associationShad are migratory fish that travel great distances between sea and river in order to reproduce. These long journeys are the source of great energy expenditure, particularly to find the most favourable spawn environment. These species are considered bio-indicators of our waterways. Their presence or absence indicates the ecological state of the water. Migratory distance was governed by energetics, behaviors, maturation, and upstream delays at dams. Individual adult migrant American shad (Alosa sapidissima) ascend the Connecticut River and spawn, and survivors return to the marine environment. Theodore Castro-Santos and Benjamin H. Letcher presented a simulation model of these behaviors.
The purpose of this model is to evaluate the effects of biological and physical variables on adult spawning success and survival. Only energy devoted to migration has been taken into account in the model. Physiology and energetics strongly affected distribution of spawning efforts and survival into the marine environment. Delays to both upstream and downstream movements had dramatic effects on spawning success. Other factors influencing migratory distance included entry date, body length, and initial energy content. Furthermore, dams alter reproductive success and have an impact on migration (delay).
This model suggests shad that spend more time in the river have greater spawning success but are more likely to die of energy depletion. Many important factors in the models presented here remain enigmatic. Perhaps the most important question is what causes shad to reverse direction and migrate downstream. Do both energetics and maturation play a role ?
Answering this question could be difficult but may be possible using, say, a combination of physiological telemetry (e.g., Hinch et al. 1996) and data on reproductive status, especially of downstream migrants. The purpose of this paper was to develop a management tool to evaluate the relative importance of biological and physical factors on shad reproduction and survival. Restoring access to spawning habitat by providing fish passage has been a central management strategy. Ecological continuum is very important to preserve species, including these migratory fish. Dams for example, were built for many reasons, at the origins in order to mill operations, and today for hydraulic energy exploitation. We have to reconsider the interest of these dams, remove those which are useless and adapt the others. This process has been under way for several years, opening the door to restoring access to the rivers.
Read the full study: Castro-Santos, T. and Letcher, B.H. (2010) Modeling migratory energetics of Connecticut River American shad (Alosa sapidissima): implications for the conservation of an iteroparous anadromous fish. Canadian Journal of Fisheries and Aquatic Sciences. 67(5): 806-830. https://doi.org/10.1139/F10-026

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.What does the future has in store for red salmon in a context of global climate change?by Camille Sestac and Amandine Tauzin
Published by the November 1, 2019 on 1:18 PM
Pacific salmon have extremely complex life histories and may be threatened by global climate change, as Peter S. Rand and colleagues investigate in their recent study.
Among all species, fishes must adapt to face disruptions caused by global climate change. Sockeye salmon (Oncorhyncus nerka), an anadromous species of salmon found in the Northern Pacific Ocean and rivers discharging into it, has a complex life cycle. As a migratory species, their energetic demands are high during spawning migration. Climate change might have important impacts on populations and their migration via variation of river discharge, increase of water temperature and decline of growth conditions. Aiming to better understand the impacts of these disruptions on the migratory performance of this species of salmon, Peter S. Rand from Wild Salmon Center teamed up with researchers from British Columbia. Their goal is to evaluate the effects of past and future trends in river discharge and temperature on the migratory performance of Sockeye Salmon in the Fraser River.
In a context of global climate change, it is crucial to understand the effects of disruptions on ecosystems and the populations living in them. Indeed, it is important to know the impacts of these disruptions on every stage of their life cycle (the juvenile freshwater period, the estuarine period, and the subadult marine period) so that we can maintain the populations stock. It’s especially important for fishery management because the fishing quota has greatly increased over the last decades and has threatened populations of Pacific salmon, particularly during their spawning migration. That’s why with three main objectives, these scientists used analysis to improve the understanding of how changes in river conditions can affect the energy use and the mortality rate in Sockeye salmon population. To do so, they used several models: one to search a link between energetic conditions of individuals and en route mortality, one to simulate the energy use during spawning migration and one to hindcast and forecast energy use by simulating fish’s behaviour and migration conditions (for more information, a tip, read the article!).
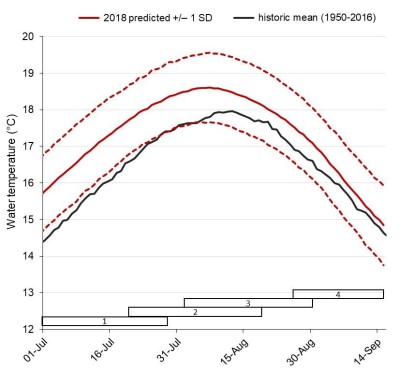
Long-range forecasts of lower Fraser river temperature during the summer of 2018Using these friendly models, Rand and his colleagues proved that energy reserves and energy depletion of early Stuart Sockeye salmon are major factors that can affect their ability to reach their spawning grounds. They also stated that energy depletion is a function of both river temperature and discharge. Therefore, this population is structured by condition-dependant mortality. Nevertheless, this group of researchers brought to light a mechanism that allows fishes to cope with some environmental variability, providing a certain degree of resilience over time. Therefore, even if energetic demands and migration mortality increase as a result of exposure to warmer temperatures, it will be compensated by reduced time travel to the spawning ground as the river flow will be lower.
However, increase of temperature means increase of diseases appearing and developing and that stress added may be a direct cause of increased mortality during migration. Finally, as if it wasn’t already bad enough for our salmons, ocean productivity can be affected by climate change and thus affect their river migration success. In fact, this can lead to a decrease of body size and body energy content. It implies that individuals will start their migration with lower energy densities and will be more likely to exhaust their energy stock before even reaching the spawning grounds.

Salmon jumping over a weirAccording to the US-Canada Commission, a 21° C temperature spike was measured on the Fraser River in 2009. However, sockeye salmon show signs of physiological stress and migratory difficulties above 19°C and from 20°C, the first signs of illness and death appear. But migration of Sockeye salmon is not only threatened by climate change. In fact, migration of salmon specially is impacted by humans or natural obstacles. Dams and weirs form large obstacles for this migratory species and can be very difficult to cross. Many studies have already proved that this kind of obstacles, even when equipped with crossing devices, delay their migration and thus jeopardize their reproduction. This can lead to a decline of the population and in some cases to its extinction, as it happened in Belgium.So, whilst some questions have been answered, it seems that more studies need to be carried out to improve our knowledge about the impact of global change which seems to be another sword of Damocles hanging over the head of Sockeye salmon.
Cited paper: Rand, P.S. et al. (2011) Effects of River Discharge, Temperature, and Future Climates on Energetics and Mortality of Adult Migrating Fraser River Sockeye Salmon. Trans. Am. Fish. Soc. 135(3), 655-667. https://doi.org/10.1577/T05-023.1
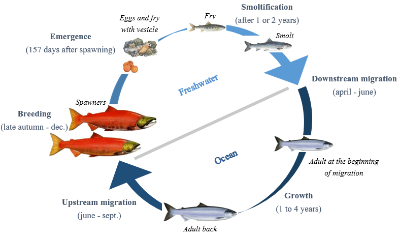
Featured images: Life cycle of Sockeye salmon by Camille Sestac, graph from https://www.pac.dfo-mpo.gc.ca/science/habitat/frw-rfo/index-eng.html , Sockeye Salmon from www.ryanvolberg.com

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Avoid predation or starvation: which strategy maximize rainbow trout juvenile survival?by Léa Bulon and Mylène Jury
Published by the September 9, 2019 on 7:12 AM
Natural populations are increasingly exposed to a range of biotic stressors, such as predators, and abiotic environmental stressors such as environmental variations (seasonality - Wingfield, 2013) or chemical pollution (Fisher et al., 2013). (We could think to grandma Margaret who throws away her bleach bucket directly into the river or grandpa George who loses all his plastic lures into the lack). The first year of life is complicated for all organisms because they are more sensitive to those kinds of stressors and their survival is highly impacted.
In temperate zones, fry are subjected to high predation during the growing season and a nutritive resource deficit during winter. This is why juveniles need to find the best way to maximize their survival and make population viability durable through the time. Predators prefers a fry-up of little fish, it is why predation mortality is higher in small fish than large (Parkinson et al., 2004). However, growth itself may impose a significant energy mobilization which can drive trade-offs between growth and other metabolic processes. If you are really interested by the topic but not by fish, we recommend you to look at the article written by Mcleod et al., 2008, about birds.
During winter, the metabolism needs some fuels like lipids and proteins to work because resources are often limited. Production of energy storage is energetically expensive, and energy contributes less to increasing their body-size.
Is it better to allocate their energy into the growing season to avoid predators or into the lipid storage to survive during winter?
To store or to grow? That is the questionStephanie Morgensen and John R. Post, scientists from Canada, are been interested in this process. They led an experiment with juvenile rainbow trout (Oncorhynchus mykiss). They developed a mathematical model to determine the energy allocation strategy maximizing the first-year survival of rainbow trout.
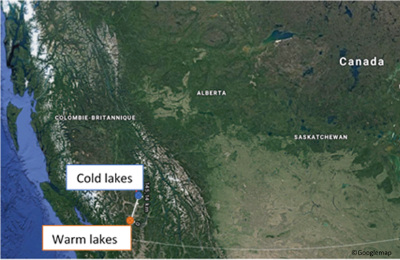
Rainbow trout juveniles are sampled from two sets of lakes in British Columbia in Canada. The first site is located on the Bonaparte Plateau and it corresponds to highly and cold lakes. The second site is located near the town of Merritt and it corresponds to low altitude and warm lakes. In warm lakes, the winter season is shorter than in cold lakes and there are more resources for juveniles.
Canada: birthplace of the rainbow troutThey found that juvenile growth is different between the two kinds of lakes: fish from the cold lakes growth more than fish from warm lakes. As we said before, production of lipid storage consumes more energy than growth and resources are more abundant in warm lakes. It is why, fish from warm lakes are more able to stock and fish from cold lakes, to growth. However, fish do not follow only one strategy. Indeed, they grow during the first part of the non-winter season and then they put their energy into the lipid storage to survive during winter. This switch between the strategies is controlled by environmental conditions and determined trout survival during winter.
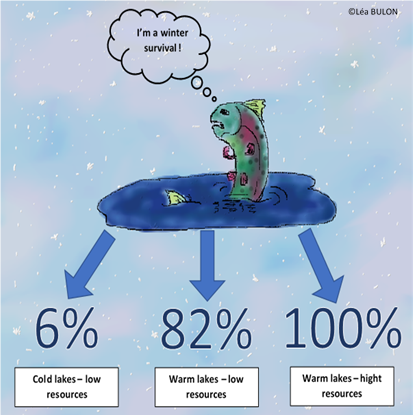
How many fish survive during winter?The juvenile survival trade-off influencing by environmental conditions such as temperature and resource availability would be important to understand population viability with the evolution of environmental conditions. Rainbow trout has been introduced into many streams and water bodies for recreational fishing because they are easy to catch and quite combative (fishing federation). They constitute an important economic interest it is why, it is one of the most studied species by biologists (INRA). This may lead to management measures to improve pisciculture conditions or to instore fishing quotas and a minimum size of capture. It could be also interesting to know if energy allocation strategies affect physiological processes like growth or reproduction.
And you what would you choose to survive during winter?

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.A damned energy loss for migratory fishes: dams!by Manon Salerno
Published by the June 10, 2019 on 9:42 AM
Many species of fish grow in the sea and breed in rivers. These migratory fish are called anadromous. When a migratory fish is ready to breed, it leaves the sea and up a river to lay watershed upstream. It will find the optimum conditions to reproduce and allow the development of its offspring. But to do so, they spend a lot of energy on the upstream and sometimes, obstacles like dams in their path does not make it easy for them. This is the case of American Shad in the Connecticut River in the United States. Since the 1970s, 4 hydroelectric dams have been built in the river. Even if they are equipped with fish ladders, these obstacles require the Shad more energy to cross them than if they were not present. We know energy availability can be a limiting factor in migration. Thus, in 1999, scientists wanted to understand energy management in these fish, especially when it is modified by the presence of such.

Any organism needs energy to perform the movements / migrations necessary for its life cycle. When they are heading into a period that will not allow them to feed (overwintering, migration), some species store energy, such as the bear before hibernating. For American Shad, this stock has to be created before migration because it will not feed during this move. First, scientists have found these are subcutaneous lipid reserves and skin constitute a special tissue for energy storage, which is rather unusual. Salmon, for example, usually mobilizes lipids from muscles and viscera. In contrast, for migration, somatic tissues (red and white muscles and skin) provide about 90% of the energy required in shad.
Although fish ladders are quite efficient at the upstream for the American Shad, it is sometimes not suitable for other species. In addition, the outmigration can also present risks of mortality (water retention, drop height etc ...). It is therefore essential to remove the dams for which their function is not provided anymore. But in the United States, the erasure of small dams often meets opposition from local communities. Even though many dams have been removed, they represent a strong historical or landscape value for the inhabitants, creating tensions between the supporters of the restoration and the local communities. This situation reminds the context existing in France, where the aesthetic and historical arguments are very powerful. Many dams are attached to mills and water plants of olden times are therefore seen as a "living historical landscape" very characteristic of their landscape. Because of the local character of each operation, an opposition not necessarily collective but influential and well directed, is enough to block some sites.

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.Temperature-dependent body size effects determine population responses to climate warmingby Alison Arraud and Laura Duran
Published by the July 5, 2018 on 1:55 PM
Up to now, neither the size nor the stage of the individual were considered to studying the population responses to climate warming. On 2014, a scientific group proposed another way to understand the temperature effects on fish populations. They improved the interaction effects of temperature-dependence with the size and the stage of fish on their energetic thresholds responses. Energetic thresholds themselves act on the dynamic of stade-structured population (e.g. parr, smolt, adult).

Flathead mullet (Mugil cephalus) - Roberto Pillon - CC BY 3.0 UnportedFinally, this study found that increasing temperature could redistribute biomass across life stages and modify the regulation of the population by reworking the intra-specific competition. Other studies have shown that high temperature during ontogenesis can accelerate the development and growth of individuals or, give individuals of smaller sizes early maturation.
This study points out the importance of taking into account the interactions between temperature and size-specific (maturing, reproduction, etc.) that will lead to a set of behavioral responses that have consequences on the structuring of a population. This is all the more important in the context of global warming.

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.Can bioenergetic models help the re-introduction of the native Rio Grande cutthroat trout in a Southwestern headwater stream?by Emmanuel Bourgoin and Aurélien Callens.
Published by the May 16, 2018 on 1:47 PM
Re-introduction of native species is far from being simple: many parameters must be accounted for! To illustrate that, we are going to take a closer look at a study made by Kalb and Huntsman (2017) on a stream in southcentral New Mexico which was deemed suitable for re-introduction of the native Rio Grande cutthroat trout (Oncorhynchus clarkii virginalis). Before re-introducing this species, researchers wanted to know if the habitat was able to sustain it. Thus, they evaluated habitat using resource selection functions with a mechanistic drift-foraging model to explain rainbow trout distributions. They studied rainbow trouts because they are present on the stream and are close relative to the Rio grande cutthroat trout, consequently all the results of this study can be extended to this native species.

Rainbow trout - Timothy Knepp/U.S. Fish and Wildlife Service - Public domainEach month, the available habitat and foraging locations were evaluated along the stream. Foraging locations were defined as the location where they could observe a foraging fish. For each foraging site, the length of the fish was estimated and physical characteristics such as discharge, focal velocity (current velocity at the head of the fish), depth, cover distance and temperature were measured on the exact fish location. These parameters were also measured on the available sites. Macroinvertebrate drift was estimated on all the locations (available and foraging). All these parameters were used in bioenergetic models which allow the researchers to estimate all the intakes of the fish (net energy intake, energy assimilated…) and all the costs associated with foraging (capturing a prey, swimming…).
First, they observed that macroinvertebrate drift was strongly season- and temperature-dependant with high values in summer and fall and low values in winter and spring. Moreover, as we must expect it, water temperature, depth and discharge were found to be seasonal parameters too. Secondly, models identified the depth as the most limiting factor for habitat selection: trout of all ages preferred habitat location with a greater depth. The most interesting thing about the models is that they can show the characteristics of the chosen habitat according to the age of the trout and the season. In fact, they showed that during the winter the smaller size-classes were more likely to choose a position closer to cover. Additionally, they highlighted that spring was the season with the greater energy intake for all the size-classes expect the 4+. Finally, drift-foraging models identified that 81% of observed trout selected positions could meet maintenance levels throughout the year and 40% of selected habitats could sustain maximum growth. Despite these last observations, the larger size-classes were energetically more limited throughout the year.
This study showed that trout population prefers deep pool habitats with slow moving water and that this stream was able to sustain a great population of rainbow trout and could consequently sustain a great native population of Rio grande cutthroat trout. However, authors warn us about the risk of hybridization and interspecific competition and suggest removing the non-native fishes first.
To answer the question in the title: yes, bioenergetic models can help to re-introduce a native species in a given environment. Nonetheless, this example is really specific: author had the chance to find and study a close relative to the native trout in the stream! The main thing to remember is that bioenergetic models give a lot of useful information on how a species uses an habitat and must be taken into account (if applicable) in the management of species.

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.