When a macaque has the choice between two lianasby Marie Delbasty and Julie Viana
Published by the May 10, 2021 on 8:00 AM
Two populations of moor macaques (Macaca maura) were studied in their natural environment in South Sulawesi, Indonesia in order to understand their use of two different habitats in karst forest.
Moor macaque is a species currently classified as "endangered" by the IUCN, mainly due to the disturbance and fragmentation of its habitat. That is why, in order to develop adequate conservation plans and management strategies, it is essential to study the patterns of habitat use in relation to the distribution of essential food resources.
Concerning the two types of habitat, they were characterized according to the vegetation present and its abundance as well as the topography and the presence of any trace of human activity.
The two habitats of this forest are in fact distinguished in two essential aspects:
The forest situated at the highest altitude with a steep slope, has few food resources but is not accessible to humans
vs.
The gently sloping forest, rich in resources but frequented by humans.
In order to carry out this study, each group of macaques was observed after having been accustomed to the presence of the scientists. The largest group consisted of about 30 individuals while the smallest group consisted of 18 macaques. The behaviour of the smallest group was studied from June to November 2016 while the largest group was studied from September 2014 to February 2015. Behavioural activities were defined as feeding, foraging, locomotion, social interactions and resting.
Although both habitats are used on a daily basis by each population, the analysis revealed that for both groups, the only behaviour that differed primarily between the two habitat types was time spent feeding. They spent more time feeding in the more food-rich forest habitat. The larger group spent more time overall in the food-rich forest while the smaller group spent more time in the food-poor forest.
The habitat with fewer resources is more of a refuge area for the macaques as they have no real predators and humans are the main threat. The larger group's use of a more productive but riskier habitat may be due to its history of provisioning, which may have allowed its individuals to have less fear of encountering humans. On the other hand, the individuals of the other group have never experienced provisioning. Another possible explanation is group size. Indeed, individuals in the larger group have less need to be vigilant because of their numbers, compared to the smaller group. In addition, the larger group might dominate the other group from a competitive point of view and thus be given priority for benefiting from resources.
In this context, macaques seem to be ecologically flexible, able to exploit the karst forest as a whole and to cope with human disturbance. It is important to protect the forest to allow the species to persist as habitat fragmentation threatens its survival. Thus, the management of this area would consist of balancing the needs of humans and macaques, and one of the solutions could be to educate local people on the protection of the species.
In a global context of loss of many species, the ideal would be to be able to leave in peace those for whom this is possible. Indeed, it seems preferable not to allow humans to access this area for the good of these macaques especially since this area is probably home to most of the remaining populations. Indeed, forest habitat with more food resources is a crucial part of the landscape for the survival of moor macaques in southern Sulawesi.
Thus, a question then arises:
would it be possible to let these macaques enjoy their habitats in peace while moving from one liana to another like Tarzan and Jane? To be continued…
Read the full study: Albani A., Cutini M., Germani L., Riley E. P., Oka Ngakan P. and Carosi M. (2020) Activity budget, home range, and habitat use of moor macaques (Macaca maura) in the karst forest of South Sulawesi, Indonesia. Primates 61, 673–684 (https://doi.org/10.1007/s10329-020-00811-8).

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.How to adapt your birdly behavior to the river flow?by Mireia Kohler Pacino and Oihana Olhasque
Published by the May 4, 2020 on 2:05 PM
The natural flow regime paradigm and the aim of study
In 1997, the natural flow regime paradigm has been established. This paradigm has become a real basement of management and basic biological study of running water ecosystems (Poff et al., 1997). This one establishes that the temporal variation in river flows requires the adaptation of structure and function of the aquatic ecosystems. To better understand this adaptation, many animals have been studied. In our case, the Cinclus Cinclus is chosen because of his large distribution in the world. We want to figure out if his behavior and energy use strategies are dictated by the natural river flow. We’ll use time-activity and time–energy budgets. In fact, it has proved to be a convenient approach to assess a bird's use of time and energy expenditure.
Using time-activity budget
Different behaviors of dippersTo answer to this question, the time-activity budget of the White-throated Dipper (Cinclus cinclus) was studied within a water basin in the Pyrenees, where natural flow regime is highly seasonal. To study the time activity budget, bird activities were categorized under four main headings: resting, foraging, diving and flying. In the study, between October 1998 and August 2001, birds activities were monitored each month using a portable tape recorder in combination with a telescope at a distance of 30–100 m. Overall, the analysis was made on 130 recordings: 62 males, 52 females, and 16 birds of unknown sex. As strategies could depend on external and river conditions, air temperature, water temperature and water column depth were measured on the behavioral surveys.
Parameters used in the study
Authors assumed that the Daily Energy Expenditure is calculated from an equation that includes time-energy budgets (obtained by incorporating time activity data), basal rate of metabolism, thermoregulation, locomotion, foraging, digestion, growth, reproduction, as well as all energy expenditures that eventually end up as heat production. The required foraging rate and the observed rate of energy gain were also calculated by dividing Daily Energy Expenditure with, respectively, the active day length for birds and the total time spent feeding by birds. Consequently, the ratio “Observed rate of energy gain” / “Required foraging rate” indicates how much faster observed feeding rates are in relation to minimum required feeding rates. For example, if birds gather food at a rate just enough to balance their energy budget then this ratio is equal to 1.
Parameters used in the DEE equationResults synthesis
The natural river flow is high during snowmelt (between April and June) and very low in summer. The behaviors are also chasing due to season: In winter our birds spend more time in foraging where food is rarely found and the water flow didn’t increase. In May, went the river flow increase, they have a rest for 70% of the day. Diving, flying and other activities showed no peculiar pattern, but there’s a relationship between water stage and time spent diving. Moreover, the ratios, observed rate of energy gain / required foraging rate indicated our birds could face high energy stress during winter but paradoxically none during high snowmelt spates when food is expected to be difficult to obtain. Unfortunately, the daily energy expenditure doesn’t seem to show any annual pattern. At this step of the study, they couldn’t find out whether Dippers use an energy strategy.
To go further....
With the actuals methods like calorimetry will be a complement to this study. To figure out, more information about dippers cycle life and potentials energy strategies. More generally, this study will serve the overwhelming challenge of maintaining native birds (especially those at risk) and more generally speaking biodiversity in human-altered rivers and streams.

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.Why should we think about cougars when planning our cities?by Amaïa Lamarins and Gautier Magné
Published by the February 3, 2020 on 2:02 PM

A puma family above the nighttime lights of San Jose - National Geographic - (photo courtesy of Chris Fust)Humans have modified 75% of earth land surface which has important consequences on wildlife. In fact, human presence and activities are perceived as a threat by animals which adapt their behaviors to avoid it. Gaynor and his collaborators’ meta-analysis showed that many species are modifying their daily activities and identified 117 diurnal mammals becoming more and more active at night. Consequently, these animals face constrained access to resources and are susceptible to shifting their diet to nocturnal prey. Thus, anthropic activities influence growth, breeding, survival and community interactions of wild animals.
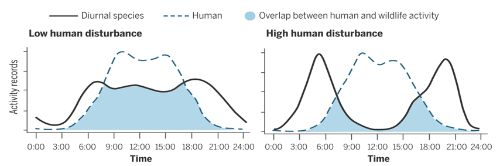
Shift in rhythmic activity of diurnal species due to human disturbance - Ana Benítez-López.In southern California, the habitat of cougars, an apex nocturnal predator, is reduced by the expansion of cities. No, we’re not talking about the rampant nightclub predators (whose habitats remain undisturbed), we’re talking about mountain lions! You’ve probably already heard about pumas roaming across big cities like Santa Cruz, California. They likely are not curious tourists hoping to take in the sites, but are rather disturbed by human activities, which cause their nighttime activity to be higher in developed areas than in natural ones. This shift increases their daily energy expenditure: because of humans, pumas need to eat around 160-190 kg of additional meat per year (for females and males, respectively)! Are there sufficient deer populations to meet these needs? Unfortunately, it seems not, since a significant number of puma attacks on cattle have been recorded.
These results, showing human-induced behavioral change for pumas, come from a recent study published by members of the Santa Cruz puma project. By wide-scale monitoring of 22 wild pumas, they were able to link their behavior with their subsequent energetic expenditures: pumas’ behavior and movement were measured through spatial GPS location data, recorded every 15min, and energetic cost of movement was estimated considering their weight and travel velocity. An interesting methodological point to note: in order to avoid underestimating the energy expenditures via GPS tracking, scientists calibrated their estimations using accelerometers. Thanks to these methods they figured out the effect of housing densities on pumas’ activity and energetic costs, taking into consideration the time of day and sex of the animal.
Indeed, they were right in taking into account these factors because, according to their findings, response to human activities differs between day and night and between males and females. During the day pumas are more likely to stay inactive, especially near urban areas. At night, being close to houses increases time spent active by 8.8% and 5.8%, respectively, for males and females. Consequently, estimated daily caloric expenditure increases by 11.6% for males and 10.1% for females in high housing density areas. Below you will find an outline summarizing these results:
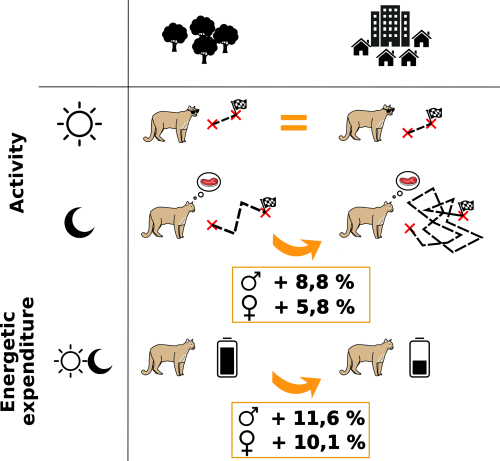
Urban development negatively affects pumas by increasing nighttime activity and energy expenditure.Such studies underline the role of bioenergetics to estimate the costs of human-induced behavioral changes but do not provide insight on global energetic allocation. Further work is needed to understand the consequences of energetic balance disturbances and identify which individual functions are affected (growth, maintenance, maturation or reproduction). Besides, human impact could be underestimated because such tracking doesn’t allow us to know if pumas get all available energy from their prey near humans; some observations reported they often have to leave their prey because they fear humans. This partial feeding would constrain pumas to hunt more prey!
Unfortunately, this is not the only human-induced threat affecting pumas. In the region of Santa Cruz and southern California, they are targeted by ranchers, resulting in political tension about their conservation. In fact, cougars have been protected since 1990. However, 98 pumas are killed each year due to depredation hunting permits. It appears necessary to ensure coexistence between urban development, human activities, puma populations and their prey. In a recent study, development strategies are suggested, such as rural residence development, to ensure landscape connectivity and conservation of parcels where pumas have been geo-located. Nowadays, no cities are expanding regarding puma, deer or other wild animals’ living areas (to our modest knowledge!). The only measures taken when pumas are too close to urban zones consist in doing nothing or frightening or relocating it, and in the worst case killing it. And if designing our lives and activities regarding nature and wildlife was the challenge of tomorrow, would you be ready?
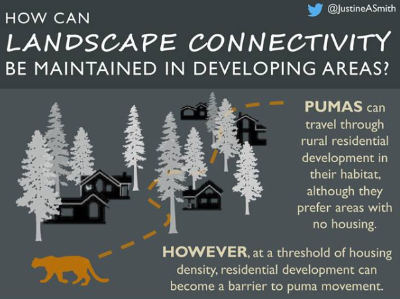
Ideal residential development maintaining pumas landscape connectivity. Graphical abstract of the paper of Smith and al 2019
This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.Albatrosses: the laziest ocean birds?by Manon Yerle
Published by the July 8, 2019 on 9:51 AM
Movement ecology is a new scientific discipline that studies the movement paradigm in the animal kingdom. The fact is: How foragers choose their hunting technics? Foragers are animals that need searching in wild food resources. Since 1960s foraging theories are studied over the world. It’s known that free-handing animals must maximize their energy gain in order to spend as little as energy possible to find and catch their preys. To better understand how this works, some scientists conducted a study about the foraging strategy of the wandering albatross (Diomedea exulans). In this study they integrate instantaneous energy budgets within the movement ecology.

Wandering albatross (Diomedea exulans) in sit-and-wait (SAW) foraging strategy. Credit: John HarrisonMaybe, you have already heard “albatrosses are always sitting on water”, “albatrosses are lazy birds” …In fact when you see one, most of the time, it’s resting on surface of the water and does NOTHING! Why is it doing that whereas it could be attacked by sharks? Moreover, water is very cold! To answer at this primordial question, the foraging strategy of the wandering albatross was studied and well characterised in the Southern Indian Ocean. Four strategies are known: foraging-in-flight (FII), area-restricted-search (ARS), sit-and-wait (SAW) and resting (RES). Strategies depend on external conditions like weather features or wind, etc… In the study, between 2002 and 2005, during brooding periods, 45 birds were tracked but prey data capture were available only for 18 foraging trips. Over 18 birds, only 5 were studied because they were complete for all data.

Albatross in FII foraging strategy. Credit: John HarrisonAuthors assumed that net energy gain equal to energy gain minus energy expenditure. Energy gain is estimated by prey capture data (stomach temperature and digestion time) and conversion factors corresponding to the diet of the wandering albatross. Energy expenditure is estimated with continuous measures of heart rates values during trips. Finally, total trip net energy is estimated by cumulating instantaneous net energy gain along the trip. Assuming external factors (wind speed and angle between flight and wind) affects foraging, they implement the flying cost model to provide energy expenditure estimates.
The most used foraging strategy is sit-and-wait because this strategy is better for the brooding period; they obtained higher net energetic gain when foraging trips are short. So, albatrosses aren’t lazy, but they are searching for food. Are their results available regarding fewer numbers of individuals? In statistical analyses it is assumed that results are available if the number of individuals is higher than 30, which is not the case here. Moreover, optimal models don’t work in wild life because there is always external factors that prevent it.
Whereas previous studies (Weimerskirch et al. 2005) identified FII strategy as the most optimal for long trips, our study identified another strategy for shorts trips, SAW. In fact, birds need to provision chicks frequently and that requires more energy than during incubation. Breeding stage defines the foraging strategy used. Another study should be managed during the incubation period implementing an instantaneous energy-budget model. Moreover, they should implement internal factors as thermoregulation that is more important in SAW for example. Now, we know how wandering albatrosses choose their foraging strategies. We can ask if SAW really is a good foraging strategy because albatrosses are more vulnerable against predators like tiger sharks.


This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.Estimating relative energetic costs of human disturbance to killer whalesby Max Davesne and Quentin Marcon
Published by the November 9, 2018 on 10:22 AM
Some predators are valued by humans, either for their ecological or aesthetic attributes, whereas others are viewed as pests. Increasingly, applied ecologists are asked to consider effects of anthropogenic activities on valued predators (Ormerod 2002). This complexity becomes especially apparent when dealing with conservation and management of cetaceans (whales, dolphins and porpoises), which are long-lived and elusive study animals. Cetaceans are also exposed to a variety of both targeted and incidental human activities in the marine environment. Nowadays, the boat traffic is always increasing as the « whalewatching » and that can cause some trouble as we don’t really knows if that disturb the ecosystem.

Boat approching a killer whale - Mike Baird - CC BY 2.0This study examined the activities of ‘‘northern resident’’ killer whales (Orcinus orca) in Johnstone Strait, British Columbia, Canada, in July and August, from 1995 to 2002. Disturbance from boat traffic has been identified as a conservation concern for this population. This study aims to test whether or not the boat presence altered whale’s activities and want to estimate the energetic cost of this disturbance for the whales.
The time-activity budgets observed with respect to boat presence were converted to rough estimates of the energetic demand of free-ranging killer whales (Kriete 1995). Only Kriete’s data from Hyak (a 4733 kg adult male) and Yaka (a 2800 kg adult female) were used, rather than values for both adult and sub-adult subjects, because data on the sub-adult female were thought to be unreliable (Kriete 1995).
Behaviour change in the presence of boats and avoidance trend and decrease in the likelihood of rubbing in the presence of boats. From Williams et al. 2006.
There is an increase of 3% in global energetic budget and a decrease of rubbing budget from 17% to 3% and for the feeding from 13% to 10%. These lost feeding opportunities lead to a substantial (18%) estimated decrease in energy intakeThis study analyzed the behavioral responses of orcas in the presence of boats. However, the model does not implement the variability between individuals. For example the stress induced by the presence of boats and the physiological differences that this may imply.
Studies demonstrated that many bird species respond to tourism presence by shortening feeding bouts (Burger et al. 1997; Galicia and Baldassarre 1997; Ronconi and St Clair 2002). This has been found also in numerous studies of terrestrial mammals, where feeding activity is easier to observe than in free-ranging cetaceans.
This study only covered one of three killer whale ecotypes. The results of this Northern residents study are difficult to extrapolate to other ecotypes (Southern residents and Migrants).

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Effects of an exotic prey species on a native specialist: Example of the snail kiteby Mathieu Finkler and Hyppolyte Terrones
Published by the September 11, 2018 on 12:34 PM
Exotic species have largely been studied over the years, their effects on native populations, their consequences... Most of the studies aim to see the competition between a native species and an exotic one. Here the study focus on the effect of an exotic species on a native predator.
Florida snail kite (Rostrhamus sociabilis plumbeus) are endangered, their populations are drastically declining in recent years. It is important to study them and to determine why their numbers are falling to implement an adapted conservation strategy.
The purpose of the study is to assess the effects of the recently introduced island apple snail (Pomacea insularum) on snail kite behavior and energetics comparing with the native prey (Pomacea paludosa).

Juvenile snail kite - Cláudio Dias Timm - CC BY-NC-SA 2.0The authors determined different parameters such as the proportion of snail dropped, the searching and handling time, the consumption rate and proportion of time in flight. Caloric intake of both species has been determined by a model (Sykes 1987) and so is the daily energetic expenditure. Caloric balance seems to be perfectly suitable in this case because the difference in intake calories could affect all the life history traits and be the cause of the fast decline of the kites.
Foraging on exotic snails led to a greater proportion of snails dropped, a lower consumption rate, a longer handling time and a lower energy balance (figure below). These conclusions are particularly true and worrying for juveniles. This results indicates that feeding on exotic snails will decrease their energy and so less energy will be available for others activities (like reproduction, growth, defence against predators...). Finally, lakes where only exotic species are present (Tohopekaliga) could form an ecological trap.
From Cattau et al. 2010Even after this study, it will be hard to conclude on an optimal foraging theory because both snail species were never found together in a lake. Therefore it could be interesting to make the same study in a lake were both species are present. Furthermore, this study has been conducted during the breeding period. During breeding period, species will need more energy to feed their offspring, to protect them, potentially leading to a greater difference in energetic balance.
Further studies may focus on the fact that kites feed on larger exotic preys (compared to native preys). Are the smallest individuals not available for kites or do kites choose to feed on larger exotic preys to compensate for their lower energetic content ?
This method could be used in others studies of trophic relationships and not only on native-exotic conflict. For example if human overfish a species, the predator of this species will have to change preys. So it will be important to calculate the energetic balance with the new prey.

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.