Time and concentration dependency in the potentially affected fraction of species: The case of hydrogen peroxide treatment of ballast waterby Marie-Adèle Dutertre and Maud Hautier
Published by the December 10, 2018 on 4:32 PM
Globalization and international trade made natural gates easier to cross for species. As a consequence, few species were able to travel long distance and settled in new habitats where they become invasive species.
More than 80% of industrialized goods in the world are transported by the oceans in container ships. In many cases, container ships is discharged in the destination port and go back empty. Whereas, the structure of this kind of ship does not allow them to travel empty and with stability. This is for a problem of stability that ballast exists. Since the 19th century, ballast with rocks was substitute with water. Before ships leave the port , water is loading in tank and at the destination port tanks are discharged.
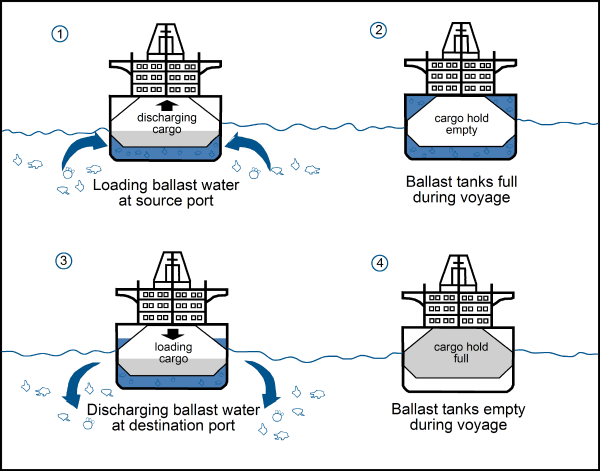
http://www.seos-project.eu/modules/marinepollution/marinepollution-c04-p05.fr.html
Ballast water transport contribute to invasive species spreading. In order to fight against exotics species, waters ballast are treated with Hydrogen Peroxide (H2O2). But there is a question : how to be sure that ballast water is effective and is not toxic for the marine environment ? In order to evaluate the environmental impact of the treatment, a study has been conducted. Three taxa has been chosen, among them, two crustacean, two algae and one rotifera : C. volutator, A. salina, E. costatum, D. teriolecta, B. plicatilis. The authors of the study consider three dimensions : Hydrogen Peroxyde concentration, the effect of the Peroxide Hydrogen on organism and Hydrogen Peroxide exposure time. In the experiment, they made the tree dimensions varied and they considered as the final aim, the mortality, the immobility and the inactivation of the organism. The results are used in a mechanistic model which is based on the description of Dynamic Energy Budget theory. The DEB theory consists of a simple set of rules that specifies how organisms acquire energy and building blocks from their environment to fuel their life cycle. It is used to rely the observed effects and the hydrogen peroxide concentration in the experiments. The DEB-tox model allows to determine ECx — Effect Concentration — : the concentration which induces a response of x% between the baseline and maximum after a specified exposure time ; and the HCx — Hazardous Concentration — : the concentration which is dangerous for x% of the population. Thanks to this values, it is possible to determine the PAF — Potentially Affected Population— with means the part of an ecosystem potentially affected by a drug concentration. The results show an interspecific response variability with means different interspecific H2O2 sensibility. Sensibility is a combination between time exposure and the concentration. The conclusion of the study is that the hydrogen peroxide is effective for treating ballast water.
Concentrations, effects and time exposure were studied there. The choice of the five species is a wise choice as a result of the representativeness of a wide selection of sensibility which allows to extrapolate this results to other species and then estimate the effect of hydrogen peroxide treatment on other species present in water ballast. Whereas the aim of the study was to assess environmental risks of hydrogen peroxide treatment, and the obtained results here cannot be used to conclude regarding as the environmental risks.
To assess more precisely the risk, it is important to consider the hydrogen peroxide degradation and its potential impact on marine ecosystem. The H2O2 is oxygenated water which would rapidly be decomposed : 2H2O2 => 2H2O + O2. In this case, the hydrogen peroxide would not impact the environment.
Furthermore, sub-lethal effects are sufficient to reduce the viability of the organisms and for that, lower concentration of H2O2 and lower time exposure are sufficient. The purpose is to neutralize exotic species with lower environmental and economic costs. Moreover, in order to reduce again the hydrogen peroxide used quantity, other studies show the efficiency of using UV, Ozone, and ultrasound for neutralizing species. The hydrogen peroxide treatment can also be used with alkaline water which allows to obtain the same result with lower concentration and time exposure.
An other option is to establish regulated areas for discharging and to filter and to purify ballast water before discharging in the environment.

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.





