Zinc pollution of our rivers: shrimp on alertby Lucille Baron and Macha Joanin
Published by the August 12, 2019 on 10:03 AM

An individual of Gammarus pulexNo, we will not make an exhaustive catalog of the pollutants that affect our streams and boredom will not settle in your heads by reading us. Instead, we have chosen to discuss the effect of one of the very present emerging pollutants zinc on Gammarus pulex, through the study of Maltby and Naylor (1990).
At present, world zinc production is over 13,000 kilotons (2018). Zinc is commonly used in metal corrosion coatings and for the manufacture of fertilizers and pesticides (European Commission, 2008b). Thanks to its physico-chemical properties, zinc melts particularly in fresh water, and it is absorbed on suspended solids and sediments (INERIS, 2014). All organisms living in these ecosystems are therefore exposed to this pollution and therefore, indirectly, we too, human.
Thus, it is important to measure the risks of this exposure on organisms and especially to know the effects of zinc on populations in the long term. For this, researchers have studied, experimentally, the energy deployed in the reproductive mechanisms by aquatic organisms exposed to zinc at different concentrations. In additions, they sought to know if exposing females during a first brood (called current) could have an effect on their second brood unexposed (called subsequent).
Gammarus pulex is a sentinel species, not only because this shrimp is abundant in fresh waters of England, but in addition it is fed with particulate matter which constitutes, in the natural environment, a large zinc stock. To measure the risks of maintaining the species and the genetic heritage of each individual under the effect of zinc, it is sufficient to study the offspring of females exposed to this compound at different concentrations. The number of individuals which hatched, and the size of each one give some indication of the energy allocated to reproduction. That's what researchers at the British Ecological Society did. If you haven’t understood anything about our attempt to explain the methods used to carry out the study experience, here is a summary diagram that you may be clearer!
Diagram of the methods used for the experiment
The results they obtained are surprising and show that exposure to zinc (even at low concentrations) significantly increases the number of broods aborted. This result is related to the decline of foods assimilated by females when they are exposed to zinc. The total energy drawn from food is no longer sufficient to sustain metabolic needs while maintaining the mechanisms of maturation and reproduction. Nevertheless, when exposed females carry her brood to term, the number of offspring of each does not seem to vary, despite of the difference in duration of exposure. So, a small criticism of the Figure which represent the percentage of broods aborted in function of the concentration of zinc (Figure 3) can be realize : we found in the control situation (not exposed to zinc), a great variability between the two categories tested. So, in science when the" control "already has significant variability, the results should be interpreted with caution thus it is difficult to conclude to a difference between the current and the subsequent.
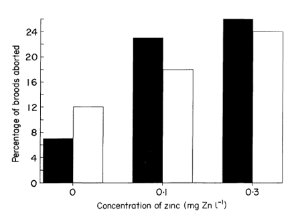
Effect of zinc on the percentage of broods aborted. Solid columns (black) represent “current” broods and open columns “subsequent” broods
The results concerning the size variation of the descendants, bring a complementarity to this analysis because the researchers observe a reduction of the size in the generations following the exposure. It is the reduction of the available energy (females stopping feedings) which as a consequence the reduction of the size of the individuals of the next brood. Also, on this point, it is unfortunate that the study does not take into account the size of the female that could have an impact on the size of the offspring (Taïr-Abbaci K., 2016).
The increase of the number of broods aborted and the decrease of the size can have a negative impact on the fitness of populations as exposure to zinc increases abortion. Also, the smallest offspring will take longer time to mature and under stress conditions, this phenomenon may be aggravated over generations and the snowball effect may strongly decreased fitness of individuals. Ultimately, these effects can have a profound impact on the entire population.
The study seems relatively far from reality since it remains experimental, in the laboratory, and is not carried out in a natural environment and therefore with real conditions of experimentation and exposure to zinc. Thus, the adaptations set up or not by the organisms and the cocktail effects (potential combined effect of different compounds) are not taken into account.
Nevertheless, the study above begins to be old, the latter dating back to 1990.Today, techniques allow to observe the embryonic development precisely and it turns out that during the different embryonic stages aberrations can appear (Bach et al., 2010). Then, it is difficult to think that abortion is the only response implemented by females when exposed to these chemical compounds.
In addition, reproductive success does not depend solely on embryonic development. It is important to consider the energy allocated to ovocyte development and the search for sexual partners to define the impact of zinc on the Gammarus pulex cohorts.
It’s possible to reduce the production of zinc with recycling it. However, the recycling of metals can sometimes be too expensive for small industries or privates companies. So, there are other ways than the installation of water decontamination mechanisms. Thus, bio-decontamination can be considered for these companies or industry but can also be useful to large industry in addition to their mechanisms for an exhaustive decontaminations and better water quality. Agriculture is also a source of metal pollution, so it is important to carry out hedgerows planting campaigns near fresh water since they have the function of absorbing a large part of the contaminants resulting from the leaching of flooded soils.

This post is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.





